

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Balanced this equation
cr2o7(2-) + fe2+ > cr3+ + fe3+ (acid)
use the concept of redu...
cr2o7(2-) + fe2+ > cr3+ + fe3+ (acid)
use the concept of redu...
Questions

Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01



Mathematics, 26.08.2020 06:01


Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01



Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

English, 26.08.2020 06:01

English, 26.08.2020 06:01


History, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

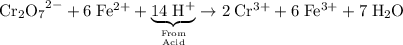 .
.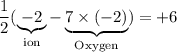 ;Fe: +2 (from the charge of the ion);
;Fe: +2 (from the charge of the ion);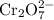 is 1. There will be two Cr atoms and hence six Fe atoms on the left-hand side. Additionally, there are going to be seven O atoms.
is 1. There will be two Cr atoms and hence six Fe atoms on the left-hand side. Additionally, there are going to be seven O atoms.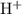 ions to produce water
ions to produce water 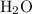 . Seven O atoms will make seven water molecules. That's fourteen H atoms and hence fourteen
. Seven O atoms will make seven water molecules. That's fourteen H atoms and hence fourteen 

