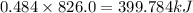Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
The heat of formation of fe2o3(s) is –826.0 kj/mol. calculate the heat of the reaction 4fe(s) + 3o2(...
Questions



Mathematics, 22.03.2021 08:30

History, 22.03.2021 08:30

Mathematics, 22.03.2021 08:30

Computers and Technology, 22.03.2021 08:30


Mathematics, 22.03.2021 08:30

Mathematics, 22.03.2021 08:30

English, 22.03.2021 08:30

Mathematics, 22.03.2021 08:30


Mathematics, 22.03.2021 08:30

Mathematics, 22.03.2021 08:30

English, 22.03.2021 08:30

Mathematics, 22.03.2021 08:30

History, 22.03.2021 08:30


Mathematics, 22.03.2021 08:30

Business, 22.03.2021 08:30

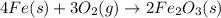
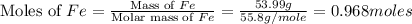
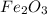
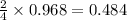 moles of
moles of