
Chemistry, 19.08.2019 23:20 regalc731ost238
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is 30.973765 amu. the masses of a proton and a neutron are 1.00728 and 1.00866 amu, respectively. enter your answer in amu's with five decimal places and no units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is...
Questions



Biology, 07.07.2019 09:30


Mathematics, 07.07.2019 09:30


Biology, 07.07.2019 09:30

History, 07.07.2019 09:30



Mathematics, 07.07.2019 09:30

History, 07.07.2019 09:30


History, 07.07.2019 09:30



Social Studies, 07.07.2019 09:30


Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

![\Delta m=[(n_p\times m_p)+(n_n\times m_n)]-M](/tpl/images/0180/0155/b6e52.png)
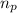 = number of protons
= number of protons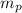 = mass of one proton
= mass of one proton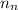 = number of neutrons
= number of neutrons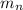 = mass of one neutron
= mass of one neutron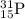
![\Delta m=[(15\times 1.00728)+(16\times 1.00866)]-30.973765\\\\\Delta m=0.27399](/tpl/images/0180/0155/689b8.png)


