

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1
You know the right answer?
Classify each of the following substances as either a strong or weak acid, strong or weak base, or a...
Questions


Business, 03.07.2019 16:30








History, 03.07.2019 16:30


Mathematics, 03.07.2019 16:30







English, 03.07.2019 16:30

Biology, 03.07.2019 16:30

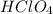 is a strong acid,
is a strong acid, 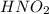 is a weak acid, NaBr is a soluble salt and
is a weak acid, NaBr is a soluble salt and 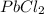 is an insoluble salt
is an insoluble salt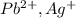 and Hg^{+}[/tex].
and Hg^{+}[/tex].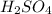 ,
, 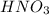 ,
, 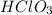 and
and 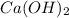 ,
, 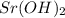 and
and 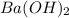 are strong bases.
are strong bases.

