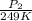The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed in a closed, evacuated container of constant volume at a temperature of 404 k. it is found that all of the ch3i is in the vapor phase and that the pressure is 72.0 mm hg. if the temperature in the container is reduced to 249 k, which of the following statements are correct
choose all that apply
a. the pressure in the container will be 104 mm hg.
b. no condensation will occur.
c. some of the vapor initially present will condense.
d. only pentane vapor will be present.
e. liquid pentane will be present.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

You know the right answer?
The vapor pressure of liquid iodomethane, ch3i, is 40.0 mm hg at 249 k. a sample of ch3i is placed i...
Questions




History, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31






English, 30.10.2019 21:31





Health, 30.10.2019 21:31

History, 30.10.2019 21:31


Mathematics, 30.10.2019 21:31

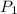 = 72.0 mm Hg,
= 72.0 mm Hg, 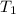 = 404 K
= 404 K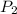 = ? ,
= ? , 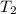 = 249 K
= 249 K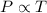 (at constant volume)
(at constant volume)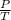 = k
= k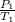 =
= 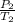
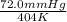 =
=