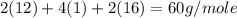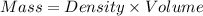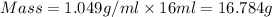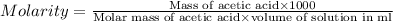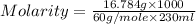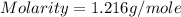Chemistry, 20.08.2019 01:20 anaroles04
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic acid at 25 ∘c in enough water to make 230.0 ml of solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic ac...
Questions

English, 07.10.2019 23:30

Mathematics, 07.10.2019 23:30

Mathematics, 07.10.2019 23:30




Mathematics, 07.10.2019 23:30

History, 07.10.2019 23:30





Mathematics, 07.10.2019 23:30


English, 07.10.2019 23:30



Physics, 07.10.2019 23:30

Biology, 07.10.2019 23:30

Spanish, 07.10.2019 23:30

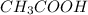 =
=