
Chemistry, 20.08.2019 02:30 notseansafe
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium constant for this reaction will
a. increase if the temperature is decreased.
b. decrease with increasing temperature.
c. increase with increasing temperature.
d. increase at some pressures and decrease at other pressures.
e. not change if the temperature is increased.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium con...
Questions


Mathematics, 14.06.2021 15:20


Social Studies, 14.06.2021 15:20


Mathematics, 14.06.2021 15:20

Biology, 14.06.2021 15:20







Mathematics, 14.06.2021 15:20




Computers and Technology, 14.06.2021 15:20

Mathematics, 14.06.2021 15:20

English, 14.06.2021 15:20

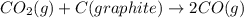
![K_{c} = \frac{[CO_{2}]}{[CO]^{2}}](/tpl/images/0180/4423/2161b.png)


