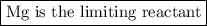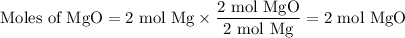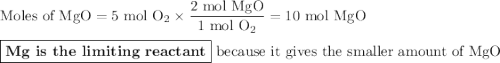Chemistry, 20.08.2019 04:20 KevinReed6444
Two moles of magnesium (mg) and five moles of oxygen (o2) are placed in a reaction vessel. when magnesium is ignited, it reacts with oxygen. what is the limiting reactant in this experiment?
mg + o2 → mgo (unbalanced)
a.
mg
b.
o2
c.
mgo
d.
both the reactants are in same proportion

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Two moles of magnesium (mg) and five moles of oxygen (o2) are placed in a reaction vessel. when magn...
Questions




History, 28.06.2019 15:20


Mathematics, 28.06.2019 15:20

Mathematics, 28.06.2019 15:20







History, 28.06.2019 15:20




Mathematics, 28.06.2019 15:20