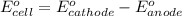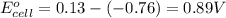Chemistry, 20.08.2019 05:10 strongl3219
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. standard reduction potentials can be found here.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and...
Questions





Spanish, 28.08.2020 14:01

Health, 28.08.2020 14:01




Mathematics, 28.08.2020 14:01

Health, 28.08.2020 14:01

Social Studies, 28.08.2020 14:01

English, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01


Mathematics, 28.08.2020 14:01

Social Studies, 28.08.2020 14:01

Geography, 28.08.2020 14:01

Business, 28.08.2020 14:01

History, 28.08.2020 14:01

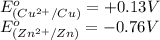
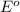 potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.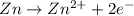
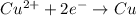
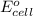 of the reaction, we use the equation:
of the reaction, we use the equation: