

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1


Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
The complete combustion of octane, c8h18, a component of gasoline, proceeds as follows: 2c8h18(l)+2...
Questions

Advanced Placement (AP), 23.11.2020 18:20


English, 23.11.2020 18:20



Mathematics, 23.11.2020 18:20

Mathematics, 23.11.2020 18:20

English, 23.11.2020 18:20

History, 23.11.2020 18:20

Mathematics, 23.11.2020 18:20

Engineering, 23.11.2020 18:20

English, 23.11.2020 18:20

English, 23.11.2020 18:20


Mathematics, 23.11.2020 18:20


Biology, 23.11.2020 18:20

Chemistry, 23.11.2020 18:30

Mathematics, 23.11.2020 18:30

History, 23.11.2020 18:30

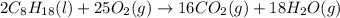
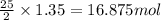 of oxygen are needed.
of oxygen are needed.

