
Chemistry, 20.08.2019 05:30 WhatTheFangirl2927
Ascientist measures the standard enthalpy change for the following reaction to be 81.1 kj : 2co2(g) + 5 h2(g)c2h2(g) + 4 h2o(g)based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of co2(g) is

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
Ascientist measures the standard enthalpy change for the following reaction to be 81.1 kj : 2co2(g)...
Questions


English, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01

Spanish, 08.10.2020 08:01

Physics, 08.10.2020 08:01


English, 08.10.2020 08:01

Chemistry, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01







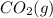 is coming out to be -410.8 kJ/mol.Z
is coming out to be -410.8 kJ/mol.Z
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0180/8814/45485.png)

![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_2H_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(CO_2(g))})+(5\times \Delta H^o_f_{(H_2(g))})]](/tpl/images/0180/8814/91e0c.png)
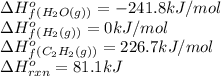
![81.1=[(1\times (226.7)})+(4\times (-241.8))]-[(2\times \Delta H^o_f_{(CO_2(g))})+(5\times (0))]\\\\\Delta H^o_f_{(CO_2(g))}=-410.8kJ/mol](/tpl/images/0180/8814/55dd7.png)


