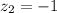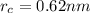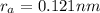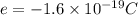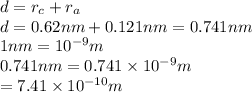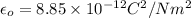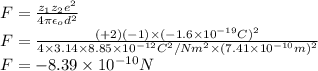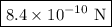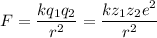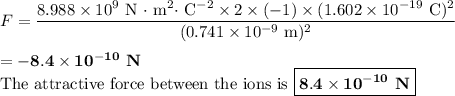Chemistry, 20.08.2019 05:30 JocelynC24
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively. (a) calculate the force of attraction between these two ions at their equilibrium interionic separation (i. e., when the ions just touch one another).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively....
Questions


Mathematics, 07.08.2019 00:20

Mathematics, 07.08.2019 00:20

Mathematics, 07.08.2019 00:20

Mathematics, 07.08.2019 00:20


Mathematics, 07.08.2019 00:20


Mathematics, 07.08.2019 00:30



Social Studies, 07.08.2019 00:30


Social Studies, 07.08.2019 00:30






Computers and Technology, 07.08.2019 00:30

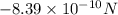 .
.