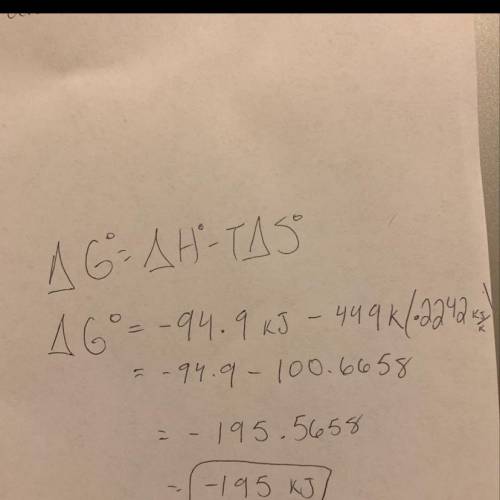Chemistry, 20.08.2019 22:20 anaroles04
Estimate δg°rxn for the following reaction at 449.0 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh°= -94.9 kj; δs°= -224.2 j/k

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Estimate δg°rxn for the following reaction at 449.0 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh°= -94....
Questions




Mathematics, 29.06.2021 19:40

Mathematics, 29.06.2021 19:40







History, 29.06.2021 19:40

Mathematics, 29.06.2021 19:40

English, 29.06.2021 19:40


History, 29.06.2021 19:40

Geography, 29.06.2021 19:40

Mathematics, 29.06.2021 19:40


Mathematics, 29.06.2021 19:40