
Chemistry, 20.08.2019 22:20 notsosmart249
Identify whether each species functions as a brønsted-lowry acid or a brønsted-lowry base in this net ionic equation. ch3coo-+hco3-ch3cooh+co32-brønsted- lowrybrønsted-lowrybrønsted-lowrybr ønsted-lowryin this reaction: the formula for the conjugateof ch3coo- isthe formula for the conjugateof hco3- is

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Identify whether each species functions as a brønsted-lowry acid or a brønsted-lowry base in this ne...
Questions


Mathematics, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20


Biology, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20

Computers and Technology, 29.01.2021 04:20

Advanced Placement (AP), 29.01.2021 04:20

Computers and Technology, 29.01.2021 04:20

Mathematics, 29.01.2021 04:20


Mathematics, 29.01.2021 04:20


French, 29.01.2021 04:20


English, 29.01.2021 04:20


Biology, 29.01.2021 04:20

History, 29.01.2021 04:20

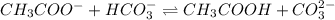
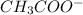 and
and 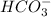 are act as Bronsted Lowry base and acid respectively and
are act as Bronsted Lowry base and acid respectively and 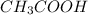 and
and 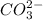 are act as Bronsted Lowry acid and base respectively.
are act as Bronsted Lowry acid and base respectively.

