
Chemistry, 20.08.2019 22:20 davionb556
Consider the following reaction where kc = 1.80×10-2 at 698 k: 2 hi (g) h2 (g) + i2 (g)a reaction mixture was found to contain 0.304 moles of hi (g), 5.07×10-2 moles of h2 (g), and 4.57×10-2 moles of i2 (g), in a 1.00 liter container. indicate true (t) or false (f) for each of the following: 1. in order to reach equilibrium hi(g) must be produced .2. in order to reach equilibrium kc must increase .3. in order to reach equilibrium h2 must be consumed .4. qc is less than kc.5. the reaction is at equilibrium. no further reaction will occur.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
Consider the following reaction where kc = 1.80×10-2 at 698 k: 2 hi (g) h2 (g) + i2 (g)a reaction mi...
Questions


Mathematics, 11.08.2021 07:50

Physics, 11.08.2021 07:50

Mathematics, 11.08.2021 07:50

Business, 11.08.2021 07:50

Mathematics, 11.08.2021 07:50

Mathematics, 11.08.2021 07:50


Mathematics, 11.08.2021 07:50

Mathematics, 11.08.2021 07:50

English, 11.08.2021 07:50

Chemistry, 11.08.2021 07:50



Computers and Technology, 11.08.2021 07:50


Mathematics, 11.08.2021 07:50

Spanish, 11.08.2021 07:50

Mathematics, 11.08.2021 07:50

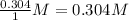
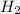 =
= 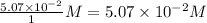
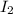 =
= 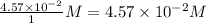
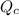 , for this reaction =
, for this reaction = ![\frac{[H_{2}][I_{2}]}{[HI]^{2}}](/tpl/images/0182/8823/7ae06.png)
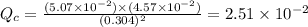
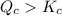 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce 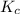 . That means HI(g) must be produced and
. That means HI(g) must be produced and 


