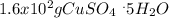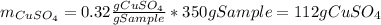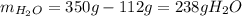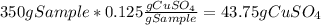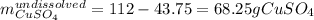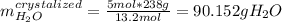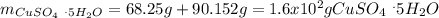Asaturated solution prepared at 70∘c contains 32.0 g cuso4 per 100.0 g solution. a 350 −g sample of this solution is then cooled to 0∘c and cuso4⋅5h2o crystallizes out. if the concentration of a saturated solution at 0∘c is 12.5 gcuso4/100 g soln, what mass of cuso4⋅5h2o would be obtained? [hint: note that the solution composition is stated in terms of cuso4 and that the solid that crystallizes is the hydrate cuso4⋅5h2o.] express your answer using two significant figures.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

You know the right answer?
Asaturated solution prepared at 70∘c contains 32.0 g cuso4 per 100.0 g solution. a 350 −g sample of...
Questions


Health, 27.07.2019 05:50






Mathematics, 27.07.2019 05:50

Computers and Technology, 27.07.2019 05:50


Computers and Technology, 27.07.2019 05:50

Mathematics, 27.07.2019 05:50

Mathematics, 27.07.2019 05:50

History, 27.07.2019 05:50

History, 27.07.2019 05:50


Geography, 27.07.2019 05:50


Mathematics, 27.07.2019 05:50

Advanced Placement (AP), 27.07.2019 05:50