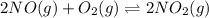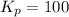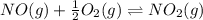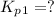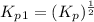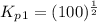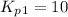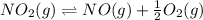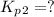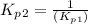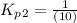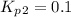Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

You know the right answer?
Find the equilibrium constants, kp, for the following equilibria, (i) no(g) + ½ o2(g) ⇄ no2(g), kp =...
Questions


History, 30.12.2019 07:31


Biology, 30.12.2019 07:31

History, 30.12.2019 07:31

Mathematics, 30.12.2019 07:31

Social Studies, 30.12.2019 07:31


Mathematics, 30.12.2019 07:31




Mathematics, 30.12.2019 07:31


Mathematics, 30.12.2019 07:31



Mathematics, 30.12.2019 07:31

Mathematics, 30.12.2019 07:31

Mathematics, 30.12.2019 07:31