
Chemistry, 21.08.2019 05:00 jessicasbss6840
[co(h2o)6]2+(aq) + 4cl-(aq) ⇌ [cocl4]2-(aq) + 6h2o(l)
concentrations at 25 degrees c
h+ 9.84368e-8
oh- 9.84368e-8
co (h2o)6^2 0.966539
cl- 1.86616
cocl4^2- 0.0334612
concentrations at 65 degree c
h+ 3.73844e-7
oh- 3.73844e-7
co (h2o)6^2 0.765375
cl- 1.06150
cocl4^2- 0.234625
a. write a k expression for this reaction (note that that liquid water shouldn’t be included in the k expression).
b. use the k expression and equilibrium concentrations on the left to determine the k value at 25 deg c. show all work for full credits.
c. what is the equilibrium constant k’ 65 degrees c?
d. compare the k’ constants; are the value difference agree or against with endo or exothermic determination?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
[co(h2o)6]2+(aq) + 4cl-(aq) ⇌ [cocl4]2-(aq) + 6h2o(l)
concentrations at 25 degrees c
h+...
concentrations at 25 degrees c
h+...
Questions

Mathematics, 16.02.2021 14:50



Mathematics, 16.02.2021 14:50

Mathematics, 16.02.2021 14:50

Mathematics, 16.02.2021 14:50

History, 16.02.2021 14:50



Advanced Placement (AP), 16.02.2021 14:50

English, 16.02.2021 14:50

Health, 16.02.2021 14:50

Law, 16.02.2021 14:50

Mathematics, 16.02.2021 14:50

English, 16.02.2021 14:50

Mathematics, 16.02.2021 14:50

Mathematics, 16.02.2021 14:50

Social Studies, 16.02.2021 14:50


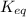 is given below.
is given below.![[Co(H_2O)_6]^{2+}(aq.)+4Cl^-(aq.)\rightleftharpoons [CoCl_4]^{2-}(aq.)+6H_2O(l)](/tpl/images/0183/8321/62682.png)
![K_{eq}=\frac{[CoCl_4]^{2-}}{[Co(H_2O)_6]^{2+}[Cl^-]^4}](/tpl/images/0183/8321/7356e.png) ......(1)
......(1)![[CoCl_4]^{2-}=0.0334612M](/tpl/images/0183/8321/e655c.png)
![[Co(H_2O)_6]^{2+}=0.966539M](/tpl/images/0183/8321/d26ec.png)
![[Cl^-]=1.86616M](/tpl/images/0183/8321/c7ee8.png)
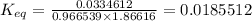
![[CoCl_4]^{2-}=0.234625M](/tpl/images/0183/8321/4e090.png)
![[Co(H_2O)_6]^{2+}=0.765375M](/tpl/images/0183/8321/d7c6a.png)
![[Cl^-]=1.06150M](/tpl/images/0183/8321/94289.png)
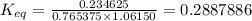
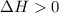 , which is positive
, which is positive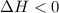 , which is negative
, which is negative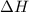 of the reaction, we use Van't Hoff's equation, which is:
of the reaction, we use Van't Hoff's equation, which is:![\ln(\frac{K_{65^oC}}{K_{25^oC}})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0183/8321/25a2a.png)
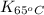 = equilibrium constant at 65°C = 0.2887886
= equilibrium constant at 65°C = 0.2887886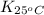 = equilibrium constant at 25°C = 0.0185512
= equilibrium constant at 25°C = 0.0185512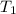 = initial temperature =
= initial temperature = ![25^oC=[25+2730]K=298K](/tpl/images/0183/8321/87ed3.png)
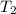 = final temperature =
= final temperature = ![65^oC=[65+2730]K=338K](/tpl/images/0183/8321/aec57.png)
![\ln(\frac{0.2887886}{0.0185512})=\frac{\Delta H}{8.314J/mol.K}[\frac{1}{298}-\frac{1}{338}]\\\\\Delta H=57471.26J/mol](/tpl/images/0183/8321/e16a7.png)


