
Liquid water at 83 c and at 1 atm flows through a heated pipe at a flow rate of 3.9 kg/s. it then leaves the pipe as steam. the water receives 12378600 j/s from the pipe. calculate the temperature of the steam leaving the pipe. the water boiling point at the pressure of the system is 100 c. thermal properties: co of liquid water: 4200 j/kg. k cp of steam: 1800 j/kg. k enthalpy of evaporation of water: 276 j/kg

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

You know the right answer?
Liquid water at 83 c and at 1 atm flows through a heated pipe at a flow rate of 3.9 kg/s. it then le...
Questions



Biology, 07.11.2019 20:31

Chemistry, 07.11.2019 20:31

Mathematics, 07.11.2019 20:31


Biology, 07.11.2019 20:31

English, 07.11.2019 20:31




Mathematics, 07.11.2019 20:31

Mathematics, 07.11.2019 20:31




History, 07.11.2019 20:31

Social Studies, 07.11.2019 20:31

History, 07.11.2019 20:31

History, 07.11.2019 20:31

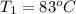 = (83 + 273) K = 356 K
= (83 + 273) K = 356 K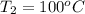 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K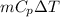
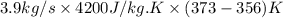
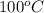 is as follows.
is as follows.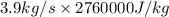
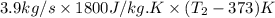
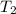 = 1552.83 K
= 1552.83 K

