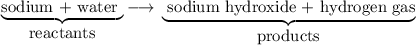Chemistry, 21.08.2019 19:30 silvajimena74
You mix solution a with solution b in a beaker. which of the following observations does not you prove that a chemical reaction has occurred?
a the beaker becomes warm.
b you see gas bubble out of solution.
c solution a, solution b, and the mixture are all yellow.
d none of the above; all prove that a chemical reaction has occurred.
in the reaction described by the word equation sodium + water sodium hydroxide + hydrogen gas
a sodium hydroxide is a product.
b sodium is a product.
c both (a) and (c)
d water is a reactant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
You mix solution a with solution b in a beaker. which of the following observations does not you pr...
Questions

History, 06.03.2020 02:10


Mathematics, 06.03.2020 02:10





Mathematics, 06.03.2020 02:11












Mathematics, 06.03.2020 02:13