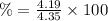Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
You know the right answer?
The solubility of a certain compound in ice-cold water is 0.16 g in 100 ml. its solubility in hot wa...
Questions

Mathematics, 18.03.2021 20:20






Mathematics, 18.03.2021 20:20

Biology, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20


Chemistry, 18.03.2021 20:20


Biology, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20



Mathematics, 18.03.2021 20:20


Mathematics, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20