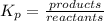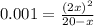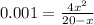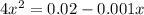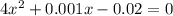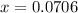Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the...

Chemistry, 22.08.2019 05:10 chrisraptorofficial
Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the expression for the equilibrium constant of this reaction.
if the initial concentration of a is 20 atm pressure, the initial concentration of b is 0 atm and the equilibrium constant kp for the reaction is .001 atm-1, calculate the equilibrium concentration of b.
i know the first part of this would be kc = [a] / [b]2 i need the second part

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1


Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Questions

Mathematics, 11.01.2020 22:31

Chemistry, 11.01.2020 22:31



Mathematics, 11.01.2020 22:31




Mathematics, 11.01.2020 22:31

English, 11.01.2020 22:31

Mathematics, 11.01.2020 22:31


Mathematics, 11.01.2020 22:31


Mathematics, 11.01.2020 22:31

Health, 11.01.2020 22:31




Biology, 11.01.2020 22:31

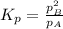 and equilibrium concentration of B is 0.141 atm.
and equilibrium concentration of B is 0.141 atm.