
Chemistry, 22.08.2019 16:10 achsahjosey
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and magnesium is added to the acid. the reaction produces a soluble salt and hydrogen gas. 2.1 write a balanced equation for the reaction. 2.2 give the name of the soluble salt. 2.3 has a physical or chemical change taken place? 2.4 2 moles of hydrogen is produced. how many moles of acid reacted?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and...
Questions








Mathematics, 18.03.2021 04:40



Mathematics, 18.03.2021 04:40


Mathematics, 18.03.2021 04:40

Mathematics, 18.03.2021 04:40



Mathematics, 18.03.2021 04:40

Mathematics, 18.03.2021 04:40


Advanced Placement (AP), 18.03.2021 04:40

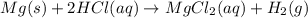
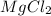
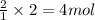 of HCl
of HCl

