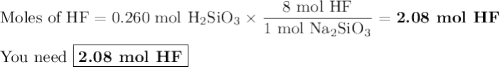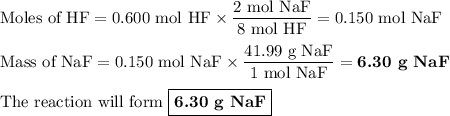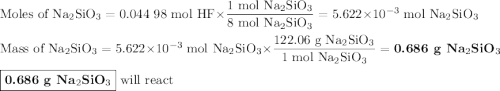Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in the glass are attacked by the hf(aq). sodium silicate (na2sio3), for example, reacts as follows: na2sio3(s)+8hf(aq)→h2sif6(aq)+2naf( aq)+3h2o(l)? a)how many moles of hf are needed to react with 0.260 mol of na2sio3? b) how many grams of naf form when 0.600 mol of hf reacts with excess na2sio3? c)how many grams of na2sio3 can react with 0.900 g of hf?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in t...
Questions



History, 29.12.2020 18:20

Mathematics, 29.12.2020 18:20





Arts, 29.12.2020 18:20




Mathematics, 29.12.2020 18:20

Physics, 29.12.2020 18:20

Business, 29.12.2020 18:20



Mathematics, 29.12.2020 18:20