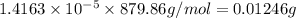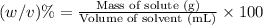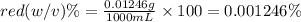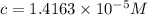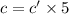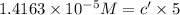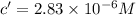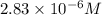Chemistry, 22.08.2019 17:20 kodakcam02
If the molar absorptivity constant for the red dye solution is 5.56×104 m-1cm-1, calculate the molarity of the red dye solution at the optimal wavelength 519nm and absorbance value 0.945
you may assume l = 1.20cm. hint: a=εlc
b. convert the molarity in part a to w/v%. show your work. molar mass of fd& c red #3 = 879.86g/mol refer to examples on next page
c. if you want to dilute the red dye solution in part a by 5 times in a single dilution step, explain in two sentences on how one should proceed with the dilution. in addition, calculate the final concentration of the diluted solution. show your work for the numerical part of this question.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
If the molar absorptivity constant for the red dye solution is 5.56×104 m-1cm-1, calculate the molar...
Questions

Mathematics, 18.06.2020 12:57

Biology, 18.06.2020 12:57


Mathematics, 18.06.2020 12:57

Mathematics, 18.06.2020 12:57

Chemistry, 18.06.2020 12:57

English, 18.06.2020 12:57

Mathematics, 18.06.2020 12:57

Mathematics, 18.06.2020 12:57

Arts, 18.06.2020 12:57

Biology, 18.06.2020 12:57


Mathematics, 18.06.2020 12:57

Mathematics, 18.06.2020 12:57


English, 18.06.2020 12:57

Mathematics, 18.06.2020 12:57

Health, 18.06.2020 12:57


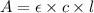
 = molar absorptivity of this solution =
= molar absorptivity of this solution =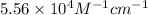
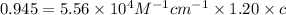
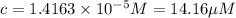
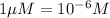 )
)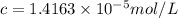
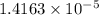 moles of red dye.
moles of red dye.