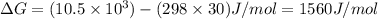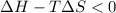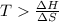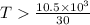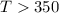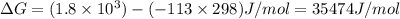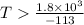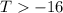Chemistry, 22.08.2019 17:30 andrew6494
6. from the values of δh and δs, predict which of the following reactions would be spontaneous at 25ºc: reaction a: δh = 10.5 kj/mol, δs = 30 j/k ∙ mol reaction b: δh = 1.8 kj/mol, δs = –113 j/k ∙ mol if any of the above reactions is nonspontaneous at 25ºc, at what temperature might it become spontaneous? (16 points)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

You know the right answer?
6. from the values of δh and δs, predict which of the following reactions would be spontaneous at 25...
Questions







Biology, 08.09.2021 02:30

Social Studies, 08.09.2021 02:30




Chemistry, 08.09.2021 02:30




Mathematics, 08.09.2021 02:30

Mathematics, 08.09.2021 02:30


Mathematics, 08.09.2021 02:30

History, 08.09.2021 02:30

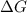 ) should be negative.
) should be negative.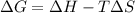 , where T is temperature in Kelvin scale.
, where T is temperature in Kelvin scale.