Chemistry, 22.08.2019 18:10 ggdvj9gggsc
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of the following changes would result in equilibrium shifting towards the products. i. increase h2 ii. decrease o2 iii. add a catalyst iv. increase the temperature v. increase h2o2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of...
Questions

Mathematics, 10.09.2019 06:10

Mathematics, 10.09.2019 06:10





Spanish, 10.09.2019 06:10



Mathematics, 10.09.2019 06:10

History, 10.09.2019 06:10



Mathematics, 10.09.2019 06:10


Chemistry, 10.09.2019 06:10

History, 10.09.2019 06:10


Chemistry, 10.09.2019 06:10

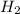 and
and 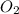 and reactant is
and reactant is