Which of the following is not equal to 1 mole of substance?
none of above
6.02 × 1023 h...

Chemistry, 22.08.2019 18:30 leannaadrian
Which of the following is not equal to 1 mole of substance?
none of above
6.02 × 1023 hydrogen molecules, h2
22.4 l of h2 gas at stp
one mole atom of carbon
6.02 × 1023 sodium molecules, na
6.02 × 1023 gold atoms, au

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Questions



Mathematics, 24.06.2020 14:01




Mathematics, 24.06.2020 14:01


English, 24.06.2020 14:01


Physics, 24.06.2020 14:01

Mathematics, 24.06.2020 14:01


Computers and Technology, 24.06.2020 14:01






Mathematics, 24.06.2020 14:01

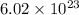 number of hydrogen molecules present in 1 mole of substance.
number of hydrogen molecules present in 1 mole of substance.

