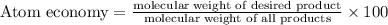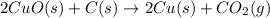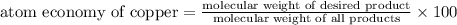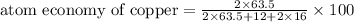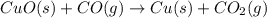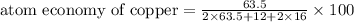Chemistry, 22.08.2019 19:10 GEEKLIFE6598
The following two reactions are possible methods for refining copper in the final step of a smelting process, i. e., getting pure copper (cu) from copper ores found in rocks. calculate the theoretical atom economy for each reaction. a. 2 cuo(s) + c(s) → 2 cu(s) + co2(g) = % b. cuo(s) + co(g) → cu(s) + co2(g) = %

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

You know the right answer?
The following two reactions are possible methods for refining copper in the final step of a smelting...
Questions

Mathematics, 27.01.2021 01:00



Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Arts, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


English, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Computers and Technology, 27.01.2021 01:00

Biology, 27.01.2021 01:00



Law, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00