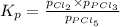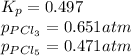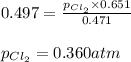Chemistry, 22.08.2019 19:20 wafflewarriormg
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)if an equilibrium mixture of the three gases in a 18.4 l container at 500k contains pcl5 at a pressure of 0.471 atm and pcl3 at a pressure of 0.651 atm, the equilibrium partial pressure of cl2 is atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)i...
Questions

Biology, 09.12.2019 01:31

Mathematics, 09.12.2019 01:31

Mathematics, 09.12.2019 01:31






English, 09.12.2019 01:31

History, 09.12.2019 01:31





History, 09.12.2019 01:31

Mathematics, 09.12.2019 01:31



Mathematics, 09.12.2019 01:31

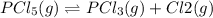
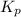 for above reaction follows:
for above reaction follows: