
Chemistry, 22.08.2019 20:10 ethanw7327
Air is compressed steadily from 150 kpa and 300 k to 600 kpa with a mass flow rate of 5 kg/s. each unit of mass passing from the inlet to exit undergoes a process described by pvls constant heat transfer occurs at a rate of 50.35 kj per kg of air flowing to cooling water. the cooling water is circulating in a water jacket enclosing the compressor. assume the potential and kinetic energy changes of the air from the inlet to exit are negligible. calculate the exit temperature of the air in k. [4 marks derive an energy balance equation for the compressor and wont determine its power input in kw.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

You know the right answer?
Air is compressed steadily from 150 kpa and 300 k to 600 kpa with a mass flow rate of 5 kg/s. each u...
Questions

History, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31

Physics, 18.01.2020 21:31


Biology, 18.01.2020 21:31

English, 18.01.2020 21:31

English, 18.01.2020 21:31

English, 18.01.2020 21:31


History, 18.01.2020 21:31





Mathematics, 18.01.2020 21:31

History, 18.01.2020 21:31

History, 18.01.2020 21:31

Physics, 18.01.2020 21:31


 = 150 kPa,
= 150 kPa,  = 300 K,
= 300 K,  = ?
= ? = constant
= constant = constant
= constant =
= 

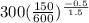

 =
= 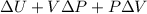


 = ?
= ?
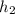 =
= 




