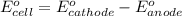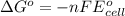Chemistry, 22.08.2019 21:30 trosclairozlynn02
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) + 2 e- → ni(s) e° = -0.23 v calculate the standard cell potential for the galvanic cell reaction given below, and determine whether or not this reaction is spontaneous under standard conditions. ni2+(aq) + 2 fe2+(aq) → 2 fe3+(aq) + ni(s)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

You know the right answer?
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) +...
Questions


Mathematics, 28.01.2020 18:01

History, 28.01.2020 18:01



Mathematics, 28.01.2020 18:01


Mathematics, 28.01.2020 18:01


Mathematics, 28.01.2020 18:01



Mathematics, 28.01.2020 18:01

Biology, 28.01.2020 18:01






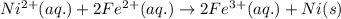
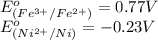
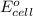 of the reaction, we use the equation:
of the reaction, we use the equation: