

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Calculate the change in the standard entropy of the system, delta s degree for the synthesis of ammo...
Questions



Physics, 04.07.2019 10:50

Computers and Technology, 04.07.2019 10:50

Physics, 04.07.2019 10:50


Mathematics, 04.07.2019 10:50


Mathematics, 04.07.2019 10:50



Chemistry, 04.07.2019 11:00

Mathematics, 04.07.2019 11:00






History, 04.07.2019 11:00

Mathematics, 04.07.2019 11:00

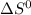 for the reaction is -198.762 J/K
for the reaction is -198.762 J/K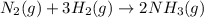
![\Delta S^{0}=[2moles\times S^{0}(NH_{3})_{g}]-[1mole\times S^{0}(N_{2})_{g}]-[3\times S^{0}(H_{2})_{g}]](/tpl/images/0189/5076/f0e35.png)
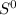 represents standard entropy.
represents standard entropy.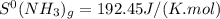 ,
, 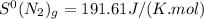 and
and 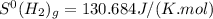
![\Delta S^{0}=[2\times 192.45]-[1\times 191.61]-[3\times 130.684]J/K=-198.762J/K](/tpl/images/0189/5076/4d0f0.png)


