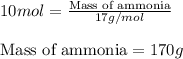Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g) + 3h2 (g) 2nh3 (g). if 140 grams of nitrogen gas is allowed to react with an excess of hydrogen gas to produce 155 grams of ammonia, what is the percent yield of this reaction?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g...
Questions

Mathematics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00

Health, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00

History, 13.11.2020 21:00


Physics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00




English, 13.11.2020 21:00



Mathematics, 13.11.2020 21:00


Mathematics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:00




 of ammonia.
of ammonia.