

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2
You know the right answer?
The enthalpy of vaporization of liquid water at 100°c is 2257 kj/kg. determine the enthalpy for apor...
Questions




English, 18.03.2021 03:00




Mathematics, 18.03.2021 03:00

Biology, 18.03.2021 03:00


Social Studies, 18.03.2021 03:00

Biology, 18.03.2021 03:00



Biology, 18.03.2021 03:00

Geography, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

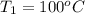 ,
, 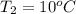
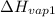 = 2257 kJ/kg,
= 2257 kJ/kg, 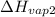 = ?
= ?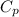 = 4.184
= 4.184 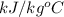
 =
= 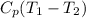
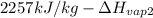 =
= 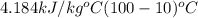
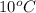 is 1880.44 kJ/kg.
is 1880.44 kJ/kg.

