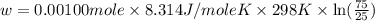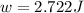Chemistry, 23.08.2019 05:30 coopyishome
Apiston chamber filled with ideal gas is kept in a constant-temperature bath at 25.0°c. the piston expands from 25.0 ml to 75.0 ml very, very slowly, as illustrated in the figure below. if there is 0.00100 mole of ideal gas in the chamber, calculate the work done by the system

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3


Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Apiston chamber filled with ideal gas is kept in a constant-temperature bath at 25.0°c. the piston e...
Questions

Social Studies, 11.12.2020 06:10

Biology, 11.12.2020 06:10



Chemistry, 11.12.2020 06:10


Advanced Placement (AP), 11.12.2020 06:10

Biology, 11.12.2020 06:10


Mathematics, 11.12.2020 06:10

Mathematics, 11.12.2020 06:10

Mathematics, 11.12.2020 06:10


Mathematics, 11.12.2020 06:10

Chemistry, 11.12.2020 06:10

Mathematics, 11.12.2020 06:20



Mathematics, 11.12.2020 06:20

History, 11.12.2020 06:20

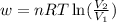
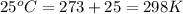
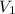 = initial volume of gas = 25 mL
= initial volume of gas = 25 mL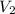 = final volume of gas = 75 mL
= final volume of gas = 75 mL