
Chemistry, 23.08.2019 05:30 brenda2512
What is the energy absorbed in this endothermic nuclear reaction 14 7 n + 4 2 h e → 17 8 o + 1 1 h 7 14 n + 2 4 h e → 8 17 o + 1 1 h ? (the atomic mass of 14 n 14 n is 14.003074 u and that of 17 o 17 o is 16.999132 u)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
What is the energy absorbed in this endothermic nuclear reaction 14 7 n + 4 2 h e → 17 8 o + 1 1 h 7...
Questions





Health, 09.06.2021 02:10

Chemistry, 09.06.2021 02:10

Biology, 09.06.2021 02:10

Chemistry, 09.06.2021 02:10



Computers and Technology, 09.06.2021 02:10

Spanish, 09.06.2021 02:10

Mathematics, 09.06.2021 02:10


English, 09.06.2021 02:10

History, 09.06.2021 02:10

Mathematics, 09.06.2021 02:10

Biology, 09.06.2021 02:10

Mathematics, 09.06.2021 02:10

English, 09.06.2021 02:10

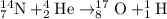
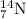 = 14.003074 u
= 14.003074 u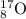 = 16.999132 u
= 16.999132 u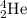 = 4.002602 u
= 4.002602 u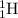 = 1.00784 u u
= 1.00784 u u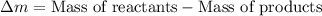
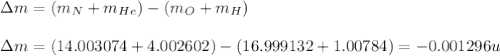
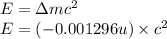
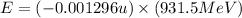 (Conversion factor:
(Conversion factor: 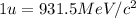 )
)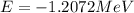 (negative sign indicates that energy is getting absorbed)
(negative sign indicates that energy is getting absorbed)

