
Calcium reacts with bromine to form calcium bromide. 2.1 which atom will lose electrons? 2.2 how many electrons will it lose? 2.3 name the element that will gain electrons. 2.4 how many electrons does this atom gain? 2.5 write down the symbol for each of the ions formed. 2.6 write the chemical formula for calcium bromide.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Calcium reacts with bromine to form calcium bromide. 2.1 which atom will lose electrons? 2.2 how ma...
Questions

Mathematics, 24.02.2021 22:00


History, 24.02.2021 22:00


Mathematics, 24.02.2021 22:00



Mathematics, 24.02.2021 22:00


Mathematics, 24.02.2021 22:00


English, 24.02.2021 22:00

History, 24.02.2021 22:00


Mathematics, 24.02.2021 22:00


Social Studies, 24.02.2021 22:00

Chemistry, 24.02.2021 22:00


Biology, 24.02.2021 22:00

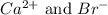
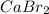
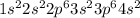
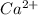 ion.
ion.![[Ar]4s^23d^{10}4p^5](/tpl/images/0191/9443/d71de.png)
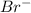 ion.
ion.

