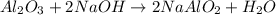Chemistry, 24.08.2019 04:30 carlosleblanc26
In the extraction of aluminium from bauxite ore, several steps are involved. in one of these, sodium hydroxide (naoh) reacts with aluminium oxide (al2o3) to produce sodium aluminate (naalo2) and water. write down a balanced chemical equation showing this reaction. explain how you can test that your equation is balanced.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3


Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3
You know the right answer?
In the extraction of aluminium from bauxite ore, several steps are involved. in one of these, sodium...
Questions




Mathematics, 11.04.2020 02:50

Mathematics, 11.04.2020 02:50




Mathematics, 11.04.2020 02:51

History, 11.04.2020 02:51




Computers and Technology, 11.04.2020 02:51

Mathematics, 11.04.2020 02:51

Physics, 11.04.2020 02:51




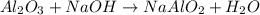
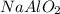 by 2 on product side.
by 2 on product side.