
Chemistry, 26.08.2019 18:00 juan01sebastian00
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen molecules and six hydrogen molecules in a closed container. assuming the reaction goes to completion, what will the final product mixture be?
a. number of nh3 molecules
b. number of n2 molecules
c. number of h2 molecules
which of the following equations best represents this reaction?
a. 42 n2 + 6 h2 4 nh3
b. 6 n2 + 6 h2 4 nh3 + 4 n2
c. n + 3 h2 nh3
d. n2 + 3 h2 2 nh3
e. n2 + h2 nh3

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1


You know the right answer?
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen mole...
Questions


Mathematics, 13.04.2021 17:40

Chemistry, 13.04.2021 17:40

English, 13.04.2021 17:40

Mathematics, 13.04.2021 17:40


English, 13.04.2021 17:40


History, 13.04.2021 17:40

Physics, 13.04.2021 17:40

Mathematics, 13.04.2021 17:40



Mathematics, 13.04.2021 17:40

Mathematics, 13.04.2021 17:40



Mathematics, 13.04.2021 17:40


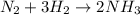
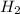 react completely with 1 molecule of
react completely with 1 molecule of 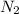 and produce 2 molecules of
and produce 2 molecules of 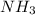 .
.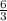 or 2 molecules of
or 2 molecules of  or 4 molecules of
or 4 molecules of 

