
Chemistry, 26.08.2019 19:00 jadenicole908
You analyze two substances in the laboratory and discover that each has the empirical formula ch2o. you can easily see that they are different substances because one is a liquid with a sharp, biting odor and the other is an odorless, crystalline solid. how can you account for the fact that both have the same empirical formula?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2


Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
You analyze two substances in the laboratory and discover that each has the empirical formula ch2o....
Questions

Mathematics, 23.02.2020 06:21


Mathematics, 23.02.2020 06:22

Mathematics, 23.02.2020 06:22



Mathematics, 23.02.2020 06:22


Physics, 23.02.2020 06:22


Mathematics, 23.02.2020 06:22

Mathematics, 23.02.2020 06:23


Mathematics, 23.02.2020 06:23


Biology, 23.02.2020 06:24



History, 23.02.2020 06:24

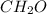
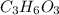 in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.
in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.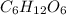 in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.
in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.

