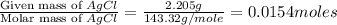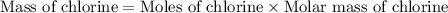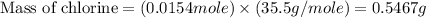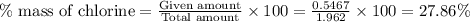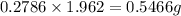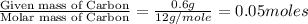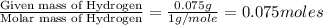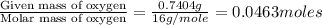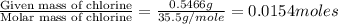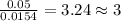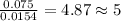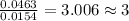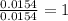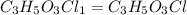Chemistry, 26.08.2019 19:30 06laurenelizabeth
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676 g h2 o. in a separate analysis 1.208 g of the compound was converted into 2.205 g agcl. the approximate molecular weight is 160. what is the empirical formula?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676...
Questions


Mathematics, 24.08.2019 05:10


History, 24.08.2019 05:10

Mathematics, 24.08.2019 05:10


Mathematics, 24.08.2019 05:10






Mathematics, 24.08.2019 05:10




Biology, 24.08.2019 05:10


Geography, 24.08.2019 05:10

Social Studies, 24.08.2019 05:10

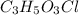
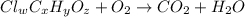
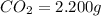

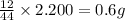 of carbon will be contained.
of carbon will be contained.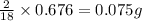 of hydrogen will be contained.
of hydrogen will be contained.