
Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. the unbalanced chemical equation for this reaction is given below: so2(g) + naoh(s) → na2so3(s) + h2o(l)assuming that you start with 36.8 g of sulfur dioxide and 20.7 g of sodium hydroxide and assuming that the reaction goes to completion, determine the mass of each product formed. g na2so3g h2o

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. the unbalanced che...
Questions


Geography, 30.11.2020 01:30


Mathematics, 30.11.2020 01:30

Mathematics, 30.11.2020 01:30

History, 30.11.2020 01:30




Mathematics, 30.11.2020 01:30

Chemistry, 30.11.2020 01:30





Mathematics, 30.11.2020 01:30




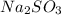 and 9.3 g of
and 9.3 g of 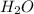 were formed
were formed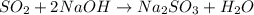
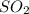 = 64 g
= 64 g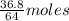 of
of 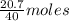 of NaOH = 0.5175 moles of NaOH
of NaOH = 0.5175 moles of NaOH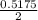 moles of
moles of 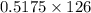 ) g = 65.2 g
) g = 65.2 g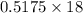 ) g = 9.3 g
) g = 9.3 g

