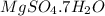Chemistry, 26.08.2019 22:10 cecelia090
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample of epsom salts is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample o...
Questions


Mathematics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01




Biology, 20.09.2020 17:01




Health, 20.09.2020 17:01

History, 20.09.2020 17:01

Arts, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Physics, 20.09.2020 17:01



English, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

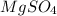 = 120 g/mol
= 120 g/mol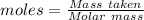
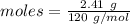
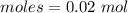
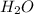 = 18 g/mol
= 18 g/mol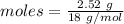
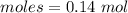
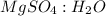 = 0.02 : 0.14 = 1 : 7
= 0.02 : 0.14 = 1 : 7