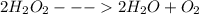Chemistry, 26.08.2019 23:30 hailiemanuel930
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a concentrated hydrogen peroxide solution. hydrogen peroxide decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). manganese(iv) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. what are the coefficients in the balanced chemical equation for this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a...
Questions

History, 17.03.2020 18:47

Health, 17.03.2020 18:47


History, 17.03.2020 18:47


History, 17.03.2020 18:47



Mathematics, 17.03.2020 18:47




Spanish, 17.03.2020 18:48







Mathematics, 17.03.2020 18:48