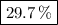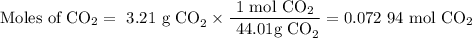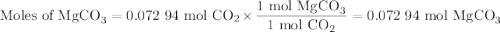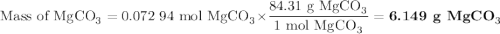Chemistry, 26.08.2019 23:30 chloiesierra29
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g) . after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 17.48 g. assum- ing that only the magnesium carbonate had decomposed, what was the percent of magne- sium carbonate in the original sample? answer in units of %.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to th...
Questions

Mathematics, 21.03.2021 05:30

Mathematics, 21.03.2021 05:30

Spanish, 21.03.2021 05:30

Chemistry, 21.03.2021 05:30


Chemistry, 21.03.2021 05:30


Mathematics, 21.03.2021 05:30



Biology, 21.03.2021 05:30


Mathematics, 21.03.2021 05:30

English, 21.03.2021 05:30

Mathematics, 21.03.2021 05:30

Mathematics, 21.03.2021 05:30


History, 21.03.2021 05:30

Mathematics, 21.03.2021 05:30

English, 21.03.2021 05:30