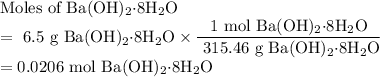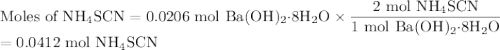Chemistry, 27.08.2019 00:20 insaneshootermo
One of relatively few reactions that takes place directly between two solids at room temperature is ba(oh)2 · 8h2o + ammonium thiocyanate --> barium thiocyanate + water + ammonia in this equation, the · 8h2o in ba(oh)2 · 8h2o indicates the presence of eight water molecules. this compound is called barium hydroxide octahydrate. what mass of ammonium thiocyanate must be used if it is to react completely with 6.5 g barium hydroxide octahydrate?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
One of relatively few reactions that takes place directly between two solids at room temperature is...
Questions

English, 19.08.2021 21:00


Mathematics, 19.08.2021 21:00


Mathematics, 19.08.2021 21:00



Mathematics, 19.08.2021 21:00


Social Studies, 19.08.2021 21:00


English, 19.08.2021 21:00







English, 19.08.2021 21:00