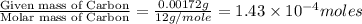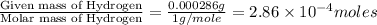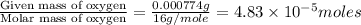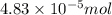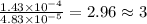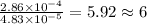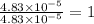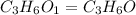Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
The characteristic odor of pineapple is due to ethyl butyrate, a compound containing carbon, hydroge...
Questions

History, 15.12.2020 04:50


Mathematics, 15.12.2020 04:50

Mathematics, 15.12.2020 04:50


Mathematics, 15.12.2020 04:50

Biology, 15.12.2020 04:50





Mathematics, 15.12.2020 04:50

World Languages, 15.12.2020 04:50


Mathematics, 15.12.2020 04:50


French, 15.12.2020 04:50

Mathematics, 15.12.2020 04:50

Mathematics, 15.12.2020 04:50

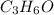
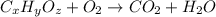
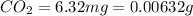
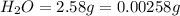
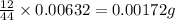 of carbon will be contained.
of carbon will be contained.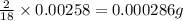 of hydrogen will be contained.
of hydrogen will be contained.