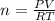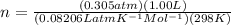Chemistry, 27.08.2019 16:20 TheSmartRey
Asolution of 62.4 g of insulin in enough water to make 1.000 l of solution has an osmotic pressure of 0.305 atm at 25°c. based on these data, what is the molar mass of insulin?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

You know the right answer?
Asolution of 62.4 g of insulin in enough water to make 1.000 l of solution has an osmotic pressure o...
Questions


Mathematics, 17.12.2019 04:31


Geography, 17.12.2019 04:31

Mathematics, 17.12.2019 04:31

Chemistry, 17.12.2019 04:31

Social Studies, 17.12.2019 04:31




Mathematics, 17.12.2019 04:31

Health, 17.12.2019 04:31

History, 17.12.2019 04:31



Mathematics, 17.12.2019 04:31



Spanish, 17.12.2019 04:31