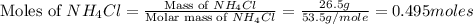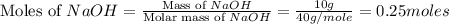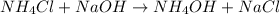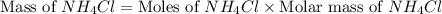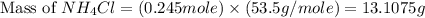Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Achemist adds 26.5g of ammonium chloride to 10g of sodium hydroxide, which follows the reaction belo...
Questions

Mathematics, 28.06.2019 19:30



English, 28.06.2019 19:30



Spanish, 28.06.2019 19:30


Mathematics, 28.06.2019 19:30

History, 28.06.2019 19:30

English, 28.06.2019 19:30

Business, 28.06.2019 19:30


Computers and Technology, 28.06.2019 19:30

English, 28.06.2019 19:30

Advanced Placement (AP), 28.06.2019 19:30

Biology, 28.06.2019 19:30

History, 28.06.2019 19:30

Biology, 28.06.2019 19:30

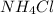 = 26.5 g
= 26.5 g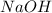 = 10 g
= 10 g